RECENT CHANGES IN REGULATORY ASPECTS OF CLINICAL TRIALS IN INDIA
Introduction:
more clinical trials, it depends on cost reduction and increase pace and production
of all R and D phases and also consists of growth impact to the favorable
regulatory climate for conducting a clinical trial in India. Various institutions are
guiding the role of clinical pharmacists in India, it includes the DCGI (drug
controller general of India) DOB (Department of Biotechnology) ICMR (Indian
council of medical research) CBN (Central bureau narcotics) RCGM (Review
committee of generic manipulation) GEAC (Generic engineering approval
committee)
Regulations in India:
collected in clinical trials is maintained for the safety and welfare of research
participate are protected.
Regulatory mechanisms:
the effect of disturbance and makes the whole system sensitive to disturbances
reducing its effect on overall system performance.
Types:
Law: A law is conducted by enforcing or controlling authority.
E.g.: the drugs and cosmetic act 1940 and rules 1945.
Regulations:
An interpretation explains to implement a law schedule.
E.g.: Y-schedule is the Indian regulation for clinical research issued by CDSCO
headed by DCGI, FDA Bhawan, and Delhi.
Guidelines:
universally accepted. it is accepted as the industry standard.
E.g.: Indian council of medical research (ICMR) guidelines, and Indian GCP
guidelines.
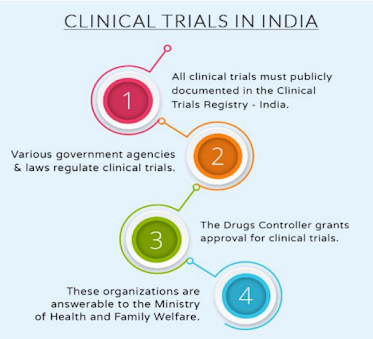
Pre-requisites of conducting clinical trials in India:
Permission from the drug controller general India (DCGI) Approval by the
ethics committee. The number of drugs approved also increased in 2015.after
three-tier reviews depending on the cases. Analyzing this we can say that scenario
of clinical trial approval has improved DCGI compared to 2014 and earlier.
Number of Clinical trials approvals in 2014 and 2015 by DCGI
Indian regulations for clinical trials
India's clinical research industry follows the drugs and cosmetic act, of
1940 recently the Indian regulations underweight progressive changes as new
drugs and clinical trial rules, 2019. Clinical trials have been registered with the
Indian council of medical registry in India (CTRI) compliance with ICH-GCP
guidelines is mandatory which describes quality assurance and safety of the
subject, SUGAM portal for the submission of SAEs has digitized the clinical trials
safety reporting methods and Indian clinical trails are moving in the right direction
in terms of automatic approvals, speedy review and response to approvals and
overall streamlining digitization and globalization of the Indian regulatory aspects
of the clinical trials.
Current drug regulatory procedures:
- Definitions of phase 1-4 tails and eliminate the phase lag
- Procedures for notifying the changes in protocol
The central drug standard control organization under the ministry of health and
family welfare describes the safety, efficacy, and quality of drugs, cosmetics,
diagnostics, and other devices in India, ministries that regulate other various
aspects of drugs such as the poisons act 1919, the pharmacy act 1948, the drugs
and magic remedies1954, narcotic drugs and psychotropic substances toilet
preparations act (excise duties) 1956, drug price control order act 2000 and
factories act 1948.
Indian clinical trials are negatively affected by noncompliance with
regulations and reports from unethical trials. Indian government authority has been
recently issued by establishing the regulating mechanisms for clinical trials. it is
based on in-depth investigation and non-governmental organization (NGO)
involvement. 1n 2019 further advanced clinical research in India routine
implementation for the clear and valid process for clinical trials. India clinical trial
regulations have been conducted on several studies and other articles are published
some other evaluation perceptions and opinions related to professionals such as
research coordinators, associates and managers have been neglected have these
professionals' awareness and opinions on NDCT rules.
They play an essential role in addressing reporting and documenting ethical
clinical trials for the investigators and ethical clinical committee and are mainly
involved in the vulnerable populations in India this review aims are explained
changes in regulations in clinical trials in India newly changed rules impact
awareness issued by 2019.
Conclusion:
On 30th September 2013, the Supreme Court’s hearing on the petition filed by the
Indore-based group, Health Forum, brought 162 ongoing clinical trials in India to a
halt as it asked the government to give details about its trial approval process. This
was a major setback but a dim ray of hope still exists as 76 new trials 64 of which
we're ongoing and 12 of which are yet to begin are approved last year as of August
2014 based on the three-parameter assessment of risk versus benefit to the patients,
innovation vies-a-visa existing therapeutic choice and unmet medical need in the
country as directed by the Supreme Court.
Qualification: B. Pharmacy
Student ID:148/0822


Comments