CHALLENGES IN IMPLEMENTATION OF GCP GUIDELINES
Introduction:
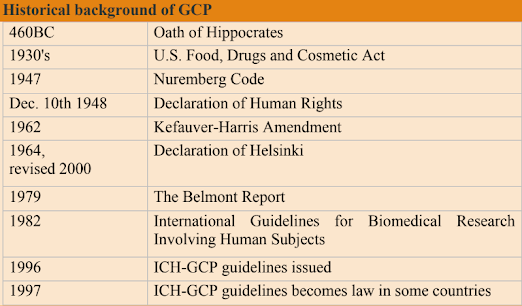
Historical background:
Participants of the 1997 conference were representatives of authorities and
pharmaceutical companies from Europe, Japan, the US, Australia, Canada, Nordic
countries and WHO. The clinical trials industry has expanded significantly since
ICH-GCP E6(R1) was published in 1996, due to an increase in globalization,
clinical research complexity and technical capabilities, the E6(R1) guideline was
flexible and allowed sponsors to implement innovative approaches to ensure
quality of clinical trials. ICH E6 got modernized with additional
recommendations so that it can better facilitate long-term implications for the
quality of clinical trials.
Challenges in the implementation of GCP:
➢ Regulatory and ethical issues:
According to CDSCO clinical trial approval and trial requirements to be carried out
by Schedule Y regulations and Indian GCP standards. The FDA
and ICH internationally promoted RBM-risk-based monitoring method,
although it is uncertain whether CDSCO will adopt a such approach,
multinational corporations conducting worldwide clinical trials and Indian
companies creating new pharmaceuticals for export will be unsure to undertake
centralized monitoring in Indian trials. Because the Indian GCP does not enable
RBM, a company that uses this approach and reduces the frequency of on-site
monitoring faces the risk of breaking regulatory standards, as continuous review
is EC's responsibility.
➢ Investigator site concerns:
On-site to centralized monitoring brings major adjustments for the investigator
site. During on-site monitoring, clinical trial monitor spends time reviewing
ICFs, IP accountability, AEs, serious-AEs, source document verification, and
site file review. As the frequency of on-site visits reduces, the site will be
required to provide some of these documents via e-mail or fax. Site staff
requires gathering previously reviewed data during the on-site visit and sending it to the
clinical trial monitor/upload on the internet portal. These lead to additional site
burden as frequent phone calls, photocopying, encrypting e-mail, scanning
documents, or e-mailing documents to the monitor. This raises issues about
privacy and confidentiality.
➢ Sponsor challenges:
Implementing ICH-E6(R2) requires significant changes in all areas - quality
systems, SOPs, technology, and team training, and at all levels - organization,
investigator, sites, CROs, and vendors. Effective centralized monitoring requires
a competent team with appropriate skills, well-defined SOPs, and electronic
technologies. RBM requires investments in validated electronic Systems like
EDC, electronic solutions for remote data access, such as cloud-based storage,
fax machines, secure websites, web portals, direct access to site files, and
electronic health records. QMS and RBM require the involvement of all functions,
with special cross-functional responsibilities for data management, statistics,
clinical trial monitoring, and medical and safety monitoring.
Conclusion:
Sponsors must raise awareness among ECs and regulators about the challenges,
opportunities, and benefits of new quality management and RBM approaches,
and proactively educate investigator sites about RBM and provide necessary
resources. Sponsor teams will also require training in novel concepts like risk-
assessment and management, setting quality tolerance limits, electronic system
validation, and analytical approach to monitoring. ICH-GCP harmonized
the standard provides public assurance that trial subjects' rights, safety, and well-
being protected and it minimizes exposure to investigational products,
improves data quality, speeds up new drug marketing, and reduces the costs to
sponsors and the public.
WRITTEN BY:
Tejaswini. Myaka
Qualification: PharmD
IDNO: 167/0922

Comments