AUTOMATION IN PHARMACOVIGILANCE
AUTOMATION IN PHARMACOVIGILANCE
Introduction
It is a process of data acquisition(entry), data analysis and giving benefit risk communication.
✓ In Data Acquisition (entry)
• Adverse event and serious adverse event collected and reported in safety database system.
• It is mostly transactional (exchange of data)
✓ In Data Analysis
• Internal data and external data surveillance occur by medical assessment and medical adjudication.
• It is mostly analytical and cognitive (intellectual)
✓ In Benifits Communication
• Risk management plan worked as aggregate reporting on the basis of health hazard evaluation and corporate safety review.
• Risk management review evaluation is analytical and cognitive.
• Aggregate reporting is mostly transactional.
• Aggregate reporting is also done by external and internal data surveillance
• Medical adjudication studies 7-15 days reporting also form aggregate reporting.
Pharmacovigilance Automation Path
• In starting period manual operations occur in pharmacovigilance after the Facilitation of database with workflow lead to automation.
• Automation is done by robotic process automation by the enhancement of natural language process and natural language generation.
• Nowadays intelligent automation taking the part lead to expansion of pharmacovigilance transaction focused with automation and intellectual theory/practices.
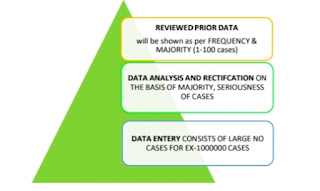
Software Automation Prioritization
• Comprehensive review of all process in form of number 1-5
• Constructing a process to distinct out the cases creating the list of cases by using automation.
• Evaluation of the cases based on relevant criteria such as time, adverse event, seriousness, number of population and complete scoring matrix.
• Use the scoring 1-5 prioritize the category of cases specific prioritization is to identify best relevant cases.
Meghana byru
Pharm.D
ClinoSol Student ID :- O48/0222






Comments