PROTOCOL WRITING IN CLINICAL RESEARCH
PROTOCOL WRITING IN CLINICAL RESEARCH
• Writing a protocol is a extremely burdensome work of a researcher.
• The researcher who writes a protocol which is to be used during the conduct of the clinical research should be a qualified,trained and have a relevant experience in the scientific field.
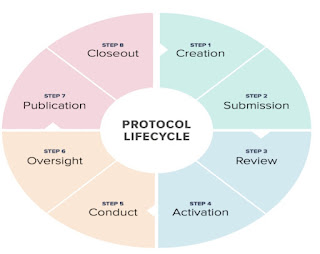
PROTOCOL
✓ It is a important document which is prepared according to the standard operating procedures,GCP,applicable regulatory requirements.
✓ It describes the every step to be carried out during clinical research and its importance.
PURPOSE OF A PROTOCOL
✓ The main objective of the protocol is to take into consideration of right,safety and well-being of the human subjects.
✓ Protocol states the risk-benefit analysis of clinical study design.
APPROVAL OF A PROTOCOL
The primary objective of the protocol should statisfy ethical principles of declaration of Helsinki.The protocol should be submitted to the ethics committee(IRB/IEC) to get approval to begin the study.It describes the eligibility criteria of the human subjects participation and their recruitment.
WRITING A PROTOCOL
1)PROTOCOL TITTLE :- Tittle should be relevant,short and clear.The protocol tittle should aim at what the study is about.
2)AIMS & OBJECTIVES :- It should have protocol identification number and date.Name and address of Investigator and sponsor and their signatures.
3)CLINICAL STUDY DESIGN :- Information regarding the investigational product.Blinding of the clinical study(double blind,triple blind).
4)INCLUSION & EXCLUSION CRITERIA :- Inclusion criteria include like acceptance human subjects based on eligibility criteria and exclusion like pregnant cases.
5)ASSESMENTS & METHODOLOGIES :- Assesment include like conducting audit trial at every phase of trial.Methodologies such as treatment studies and observational studies.
6)STATISTICAL ANALYSIS:- Theses include like maintenance of drug accountability forms.Verifying null and alternateive hypothesis.
7)SCHEDULE OF VISITS :-
i. INITIAL VISIT
ii. PERIODIC MONITORING VIST
iii. CLOSE – OUT VISIT
CONCLUSION
The conduct of clinical trial mainly implies on the prepared research protocol it should be precise,understandable,erroe-free.It will clearly distinguish the responsibilities of investigator,sponsor and human subjects.It is used to test the hypothesis of study report.
REFERENCES
1)https://www.ncbi.nlm.gov/pmc/article/PMC5198475/
2)https://www.nuventra.com/resources/blog/best-practices-clinical-study-protocol-writing/
3)https://www.kolabtree.com/blog/how-to-write-a-clinical-trial-protocol/
SIDDAM SRAVANI (B.Pharm)
Clinosol ID :- 019/0121.

Comments