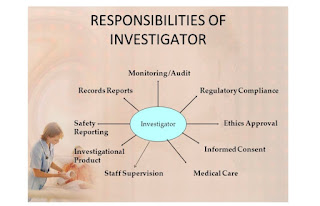
Roles and responsibilities of Investigator
Who can be as a Investigator?
A qualified person who is responsible for all Clinical trial related decisions.
✓ If the clinical trial is conducted the Investigator plays a crucial role in the team,so called as Principal Investigator.
✓ Sub-Investigator: a member of Clinical trial team, designated and supervised by principal Investigator to perform trial related duties and functions.
Roles and responsibilities of Investigator:
Qualifications and Agreements of the Investigator:
✓ Qualified by education, Experience and training
✓ Should provide the proof of such qualifications through the Curriculum Vitae
✓ Should be familiar with the terms of Protocol, Investigator’s Brochure and the Investigational product
✓ Should be familiar with the terms of Good clinical practises and Regulatory Authorities.
Adequate Resources:
✓ The investigator should know that all the person assisting the trials about the protocol
✓ Should know about the required Qualified staff involved in the trail.
✓ Should be able to complete trial in the expected time
✓ Should take the required number of subjects for the trial completion
Medical care of Trial subjects:
✓ Responsible for all trial-related decisions of medical and dental
✓ Ensure that medical care is provided to a subject for any kind of adverse events
✓ Should inform a subject when medical care is needed for illness happening at the ad mist of the trial.
✓ Should be able to inform the subject’s primary physician about participation into the trial.
✓ Subject is not given any reason for withdrawing in the trial
Institutional review board/ Independent Ethics Committee communication:
Before performing a trial should have:
✓ Trail protocol
✓ written informed consent
✓ subject recruitment procedures
And they should also provide:
✓ The current copy of the Investigator’s Brochure.
✓ If IB is updated during the trial, the Investigator/institution should supply a copy of the updated Investigator’s Brochure to the IRB/IEC.
Compliance with protocol
✓ Conduct the trial in accordance with the protocol agreed by sponsor and Regulatory authorities.
EXCEPT….
In emergency condition to eliminate immediate hazard to trial subjects or if change involves only logistical or administrative aspect of the trial (e.g., Change of monitor or change of telephone Number
✓ If there are changes in the protocol it should be submitted in the form of Protocol amendment.
Investigational product (IP)
✓ Maintaining the records.
✓ Ensure proper storage
✓ Ensuring the IP specified by the protocol
✓ Keeping list of Randomized codes
✓ Explaining correct use of IP to the participants
✓ Reconciling of IP received
Informed Consent form:
✓ It is the voluntary consent given by the subject whether to participate or not in the trial after explaining all the risks and benefits of the undergoing trial
✓ It is the first document to signed by the Subject which is prepared by the Sponser and handled by the Investigator
There are three situations to be discussed:
In case of emergency situations
✓ When prior consent of the subject is not possible and the subject’s legally acceptable representative is not available But should be clearly mentioned in the protocol and signed by IRB/IEC committee
Randomization Procedures and Unblinding
✓ The Investigator should conduct the trial accordance with protocol and the code is also broken with the compliance of it.
✓ If suppose the trail is blinded the sponsor should be clearly explained about the premature blinding
Records and Reports
✓ Accuracy, completeness, legitabilty and timeline of the data should be reported by Investigator
✓ Data which is in entered in the Case report for should be according to the source documents.
✓ Any changes in the trails should be documented, Initialised (Audit Trail)
Progress Reports
✓ Should submit written summaries of the trial related documents frequently or annually to the IRB/IEC commitees.
✓ Report written records to the sponsor and IRB/IEC including with significant changes and the risks involved.
Safety Reporting
✓ The Investigator should report regarding Serious Adverse effects to the Sponser EXCEPT
✓ The subjects should be assigned with the codes.
✓ Investigator should supply the sponsor and the IRB/IEC with related Autopsy reports
Premature Termination Or Suspension Of The Trial
If the trail prematurely terminates?
✓ Inform the trial subjects for the follow up treatments and therapy
If the Investigator suspends a trial ?
✓ Should inform the IRB/IEC about the suspension or the premature termination.
If the sponsor terminates or suspends a trial?
✓ Should provide a detailed written explanation of the termination or suspension to the
IR/IEC committee.
If the trail is terminated by IRB/IEC terminates ?
✓ The investigator should give the detailed documents about the premature termination and suspending of the trial to the sponser
Final report by Investigator
After the trail documents should be reported to the IRB/IEC and regulatory authorities
Conclusion:
The Investigator Main theme lies with the protection of rights, safety and well-being of the
Subjects involving in the clinical trials. The investigator sum-up all the data to be submitted
To the sponser and the Regulatory bodies/IRB/IEC committee
References:
ICH GCP E6 guidelines
I pay my sincere thanks to the mentor and founder Mujjebuddin sir of Clinosol research for driving me throughout of this blog and management for supporting me.
S.SAIPREETHI
M.PHARMACY


Comments