Audits in Clinical Research and How to Prevent Audit Findings
Audits in Clinical Research and How to Prevent Audit Findings
According to ICH GCP E6 Audit is defined as “Systematic and Independent examination of trial related activities and documents to determine whether the evaluated trial related activities were conducted and data were recorded, analysed and accurately reported according to the protocol, sponsor SOP’s, GCP and applicable regulatory requirements”
Main objective of an Audit
To evaluate the trial conduct and compliance with quality systems, SOP’s, GCP, protocol and other regulatory requirements.
Auditors are independent of the clinical trial/sponsor/CRO/site.
Documents to be Audited
Organization and personnel responsibilities and functions.
Qualification, training and adequacy of all the lists of investigators, monitors and other staff, Quality management systems.
Investigational medicinal product (IMP).
IRB/IEC (responsibilities, functions, operations, procedures, records, approval forms).
Essential documents (IB, Signed protocol and amendments, advertisements, ICF, financial aspectsss, subject enrollment log, subject identification list, CRF’s, SAE reportings, randomization, shipping records, accountability, de-coding procedures, monitoring plan and reports).
Bio-analytical laboratories, Computerized systems, Statistical data.
Types of an Audit
1. Sponsor audit of investigative site
2. IRB audit of investigative site
3. System audits
4. Clinical laboratory audit
5. Clinical study report audit
6. Validation of the computer system
Audit Findings
Audit findings are the results of an audit which are written in an audit report. The audit findings are based on evidence about how the process of clinical trials are conducted against the criteria of an audit.
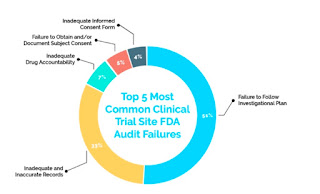
Top five clinical trial Audit Findings:
The FDA reported 36% of failure rate in clinical trial site inspection from last five years. This is the list of 5 most common clinical trial site FDA audit failures.
1. Failure to Follow Investigational Plan (51%)
a) Missing consent documents.
b) Unable to produce protocol required procedures.
c) Missing documentation of IRB review.
d) Changes made in protocol without IRB approval.
e) No documentation of reason for missed tests, schedule change of visits.
Solutions:
Always read the protocol before starting a trial to make sure it is realistic.
Do not confuse research and treatment.
If protocol deviation occurs more than once, check the protocol thoroughly in case if any modification is required.
Always document each and every changes occur from the original plan.
2. Inadequate and Inaccurate Records (33%)
a) Differences between CRF’s and medical records.
b) Incomplete CRF’s.
c) No documentation of PI review of CRF’s.
d) Improper error corrections.
Solutions:
Read the CRF’s carefully before starting the study to make sure CRF captures necessary study data.
Set up a regular research team meeting time to review the study data.
Avoid messy CRF build up.
3. Inadequate Drug Accountability (7%)
It focuses on record keeping of drugs used in research a failure of this includes absence or inadequacy of records on receipts, poor prescription practices, preparation, use, and/or disposition of the investigational drug/product.
Solutions:
Document reminders to subjects to complete pill entry diaries.
Phone call before and after visits.
Letter should be obtained for the return of the drug.
Improve the written practices.
4. Failure to Obtain and/or Document Subject Consent (5%)
a) No source documentation of consent process.
b) Consent not dated by the subject.
c) No re-consented documents.
5. Inadequate Informed Consent Form (4%)
a) Incorrect ICF version.
b) No HIPPA authorization.
c) Original consent form missing.
d) Check boxes on ICF left blank.
e) Signature not initialed by the subject.
Solutions:
Include a note regarding consent process (how it was conducted, whether questions were asked/answered)
Signed copy of ICF should be provided to subject.
Before starting consent process, always make sure to check that you have latest approved version of ICF document from IRB.
Never fill out consents in advance prior showing the ICF to the subject (ICF should be blank).
Audit findings can also be prevented by building e-Regulatory and e-Source databases. Digital transitions will be really beneficial to avoid audit findings.
1. Global Dashboards: These ensure even a single expiration date or due date for a document is not missed.
2. Remote Monitoring and Access Control: This will help in understanding who is accessing what and when.
3. Automatic Audit Trials: They easily track when and who created, edited and viewed a document or an entire binder.
4. Secure Cloud Based Document Storage: All study documents stored in a secure centralized location available 24/7 from anywhere in the world.
5. Direct EMR/EHR Integration: They capture source documents directly from EMR/HER.
6. Grant access to FDA Auditors: They grant easy access to certain files to FDA Auditors.
7. Part-11 Complaint Signatures: e-signatures are gaining momentum replacing wet signatures in clinical trials.
Conclusion
The best way to prevent the audit findings or audit failures is to strictly follow the protocol right from selecting of the subjects till the close out of the clinical trial. All the procedures should be followed strictly in compliance with investigational plan/protocol. The next important step in preventing audit failures is Documentation. Every issue/procedure/changes in protocol/SAE’s that happens during the clinical trial should be strictly documented with signature and date. We can also use several electronic databases to store the documents. By following the protocol strictly adhered with GCP, SOP’s and regulatory requirements throughout the clinical trial, the auditing findings/failures can be prevented or reduced.
By
Nalini
Mehvish Hussain


Comments
clinical Research Training
clinical Research Training in Hyderabad
best clinical research