BIAS AND ITS CONTROL IN RANDOMISED CLINICAL TRIALS
BIAS
AND ITS CONTROL IN RANDOMISED CLINICAL TRIALS
BIAS
- Any
repeatable error in the study design, conduct, or analysis of a study that
encourages one outcome over others. The disadvantage of bias is investigators
will come to the wrong conclusions about the beneficial and harmful effects of
medication.
HOW DOES BIAS EFFECT RESEARCH?
Bias
in research can lead to wrong conclusions. Such studies can result in wrong
clinical practice and they can sometimes cause harm to the patient.
TYPES OF BIAS IN CLINICAL TRIALS-
• Selection bias –It is an experimental error that
happens when the participant pool data is not representative of the target
population.
• Performance Bias - may occur by both the
researcher as well as the participant due to the knowledge on the allocation
• Detection bias –means systematic differences between
groups in which outcomes are determined.
• Attrition bias – A type of selection bias that
results from changes in the experimental result between study groups in the
number and the way participants are lost from the study.
• Reporting bias – Occurs when dissemination is
influenced by the nature and direction of results, for instance in systematic
reviews.
HOW TO MINIMISE BIAS IN CLINICAL TRIALS-
Bias
can be minimized by using masking or blinding which means one or more parties
like the subject, investigator, the sponsor is unaware of the treatment drug
and these studies are designed to avoid bias.
SOURCES OF BIAS-
• Investigator
• Participant
• Literature
• Statistician
• Instruments
WHERE BIAS CAN HAPPEN- Bias can arise at three steps of the study
- During initial enrolment of the
participants
- During the implementation of the study
- During the analysis of findings.
HOW TO AVOID BIAS IN RESEARCH?
• Use multiple people to code data
• Verify the data with more sources
• Check for other explanations
• Review findings
• Use third-person opinion
WHY SHOULD BIAS AVOIDED IN RESEARCH-
Bias
can result in a false conclusion and giving the wrong idea or impression.
Therefore it is not acceptable to conduct research.
TECHNIQUES TO AVOID BIAS –
- The randomization of subjects.
- Double blinding of subjects as well as
investigator.
- Monitoring clinical trial.
- Checking source documents.
- Verification of source documents.
- Clinical data management.
- Procedures of quality control and
assurance.
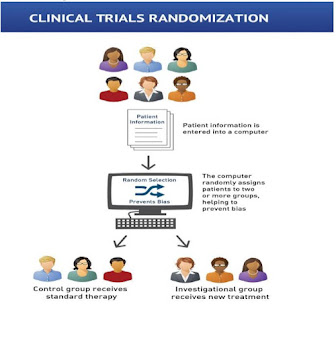
RANDOMISATION – Process of assigning trial subjects to treatment or control groups
using an element of chance to determine the assignments to reduce the bias.
WHY RANDOMISATION?
• To ensure comparability of the two arms
regarding known and unknown factors.
• Avoid selection bias.
• 3. Helps to design standard statistical
analysis
• To increase the predictive value of the study.
TYPES OF RANDOMISATION-
• Simple randomization
• Block randomization
• Stratified block randomization
• Unequal randomization
SIMPLE RANDOMISATION –
• The basic method of simple randomization is flipping a coin.
• Computer-generated sequence.
• The randomization approach is simple and easy to implement in clinical
research.
• In large clinical research, i.e., (n
simple randomization can be applied to generate similar numbers of subjects
among groups.
• However, randomization results could be problematic in small sample size
i.e.,(n˂100) clinical research, resulting in an unequal number of participants
among groups.
BLOCK RANDOMISATION-
•
The block
randomization method is intended to randomize subjects into groups that end in
equal sample sizes.
•
Used for small
studies to maintain a good balance among groups.
STRATIFIED BLOCK RANDOMISATION-
• The stratified randomization requires the need to control and balance
the influence of covariates.
•
Typical examples of
such factors are age group, the severity of the condition, and treatment
center.
UNEQUAL RANDOMISATION-
When two or more
treatments under evaluation have a price difference. The Substantial cost
savings can be achieved by adapting a smaller randomization ratio such as a
ratio of 2:1, with only a modest loss in statistical power.
CONCLUSION-
The benefits of
randomization are numerous. The use of online randomization was effectively
demonstrated in this article for benefit of researchers. Simple randomization
works well for giant clinical trials and for tiny to moderate clinical trials
without covariates, the utilization of block randomization helps to attain the
balance. For small to moderate size clinical trials i.e.,( n˃100) with several
prognostic factors or covariates, the adaptive randomization method could be
more useful in providing a means to achieve treatment balance.
-By
K. Bindu Priya
R. Rikitha
Students
at Clinosol research pvt ltd.






Comments