Corrective And Preventive Action
Corrective And Preventive Action (CAPA) in the pharma industry – an approach
Abstract
When it comes to implementing corrective and preventive action (CAPA) plans in clinical trials, we consistently hear similar frustrations from members of the research community year after year. They typically identify and implement corrective actions, but identifying and implementing preventive actions, which aim to minimize risks to other studies, is still somehow getting lost in the shuffle. Even with all the regulations, related guidelines, training, company policies, procedures, and quality systems in place, the same issues keep reoccurring in different trials. This happens for several reasons, namely because, when it comes to root cause analysis (RCAs), the correct root causes often aren't identified beyond the individual study being reviewed. More often than not, the focus is solely on the tactical issue or issues at the study level, rather than also at the system level, or across studies. Consequently, issues are frequently only resolved within the individual study where they were identified, tactically, and not further addressed with preventive measures for other, ongoing, and future clinical trials. A key reason RCAs are frequently not incorporated at the system level is the lack of available data on issues across trials. This makes pinpointing the systemic root cause(s) very difficult and frustrating for organizations that see the same issues and challenges believed to have been previously addressed pop up across their portfolio in other trials.
Keywords: Corrective and Preventive Action (CAPA), Root Cause Analyses (RCAs).
Introduction
Corrective Action: Action to eliminate the cause of an identified problem or deviation. Corrective action is taken to prevent a recurrence.
Corrective and Preventive Action (CAPA): The processes have taken and process improvements initiated to eliminate causes of nonconformities or other undesirable situations.
Preventive Action: An action to eliminate the cause of a potential non-conformity or other undesirable potential situation. Preventive action is taken to prevent occurrence.
Root Cause Analysis: A class of problem-solving methods used to identify the root causes of problems or events.
Regulatory Guidelines
The device GMP covers CAPAs in the Code of Federal Regulations (CFR) Title 21 Part 820.100 Corrective and preventive action, which state (1):
FDA investigators utilize the following instructions from an inspectional guide to inspecting a firm's CAPA system:-
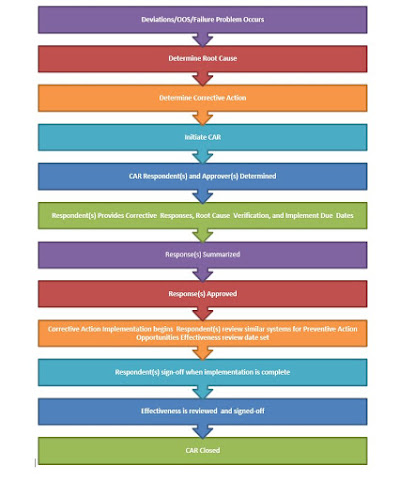
CAPA Process Map
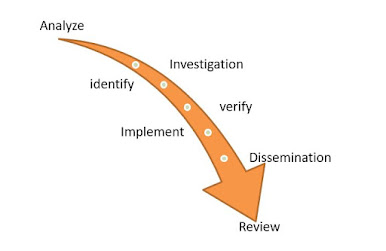
Learn about other CAPA phases
- Analyze: Perform a root cause analysis of the problem.
- Identify: Pinpoint the problem or potential problem.
- Investigate: Research the problem.
- Implement: Execute the action plan and track action item, responsibilities, and completion status
- Verify: Assess the magnitude and impact of the problem.
- Dissemination: Create a list of required actions and due dates to eliminate the problem and prevent a recurrence.
- Review: Verify and assess the effectiveness of the plan. Make changes to the plan if needed.
CAPA Sub-Systems:
Procedure:
1). Research personnel will be aware that a Corrective and Preventative Action (CAPA) Plan can be triggered in a number of ways that may include:
- During a Site Initiation Visit (SIV) or Routine Monitoring Visit (RMV) when a monitor reports that a site requires a "for cause" or "not for cause" audit.
- During an internal audit
- During an FDA audit
- Preventative actions for potential problems and issues
2). To minimize the need for a CAPA, research personnel will maintain ongoing training requirements necessary to work in research and comply with the Standard Operating Procedures (SOPs) of the CRC.
3). Research personnel will recognize that a CAPA plan can include more than one activity and issue whereas, a Note to File (NTF) usually addresses one problem or issue that has occurred.
4). Research personnel will adhere to the following principles when developing and executing a CAPA plan:
- The plan should make sense.
- The plan should be easily implemented and managed.
- The plan must be systematic and measurable. It must be proactive and able to correct problems at all levels of the research site. It should be easy to use and track the progress of the action to be able to show results that are working.
- The plan should account for more than a reaction to problems. It should have the potential to improve the efficiency and effectiveness of the clinical trial.
5). Research personnel will complete a CAPA Plan Response Form (Appendix JJ) that includes the following when preparing a CAPA plan:
- Identify: Pinpoint the problem or potential problem.
- Evaluate: Assess the magnitude and impact of the problem.
- Investigate: Research the problem.
- Analyze: Perform a root cause analysis of the problem.
- Act: Create a list of required actions and due dates to eliminate the problem and prevent a recurrence.
- Implement: Execute the action plan and track action item, responsibilities, and completion status
- Follow-up: Verify and assess the effectiveness of the plan. Make changes to the plan if needed.
Getting to the root of the problem:
In 2018, the International Council for Harmonization (ICH) of Technical Requirements for Pharmaceuticals for Human Use issued an addendum to update its guideline for good clinical practice (GCP). Taking the evolution of new technology and the use of e-clinical tools into consideration, ICH E6 (R2), as the addendum is commonly known, encourages life sciences organizations to pursue innovative approaches for conducting clinical trials, specifically quality by design (QbD) and risk-based quality management (RBQM). ICH E6 (R2) also sets the expectation that sponsors and contract research organizations (CROs) have systems in place to prevent non-compliance and efficiently manage significant deviations with appropriate CAPAs. However, regulatory findings worldwide consistently reveal that the CAPAs that sponsors, CROs, and investigators have in place is insufficient because there isn't enough focus on preventive measures at the system level, across their studies. In clinical research, quality issues that come up must be addressed and resolved in a timely, effective, and regulatory compliant manner. When a problem is minor and can be immediately addressed with a sufficient solution, then the quality issue can be easily resolved. However, sometimes issues are more significant and complex and require a CAPA plan. When developing a CAPA plan, the quality issues that need to be addressed may be isolated or more broadly represented across a clinical trial or entire R&D program. Either way, these issues can create risks to clinical trial data integrity and or patient safety.
References:
Code of Federal Regulations, Title 21, Food and Drugs (Government Printing Office, Washington, DC), Part 820.
Code of Federal Regulations, Title 21, Food and Drugs (Government Printing Office, Washington, DC), Part 211.
FDA, Current Good Manufacturing Practice Requirements for Combination Products
FDA, Guide to Inspections of Quality Systems (Rockville, MD, Aug. 1999).
ICH (2005). The harmonized tripartite guideline, Quality Risk Management, Q9, version 4, November 2005.
Tonya W (2013). Corrective and Preventive Actions, A five-step approach.
MarliseGygerMs (2012). ETH, Corrective Actions and Preventive Actions, Quality and GMP, June.
Kimberly LW (2008). Food and Drug Administration, Division of domestic field investigations, the office of regulatory affairs, Corrective and Preventive Action-Background, and examples.
By:
RAKSHANDA TALAT
M. Pharmacy (Deccan School Of Pharmacy, Hyderabad)
rakshandatalat5@gmail.com
Intern at Clinosol Research Pvt. Ltd.





Comments