CRF DESIGNING
CRF DESIGNING
DEFINITION : Case report form (CRF) is a printed ,optical or electronic document to record all protocol required information to be reported to sponser on each trial sub jects as per ICH guidelines .
Source documents first captured information ,record s are Collected from each participants subject of clinical trial are capture into CRF.
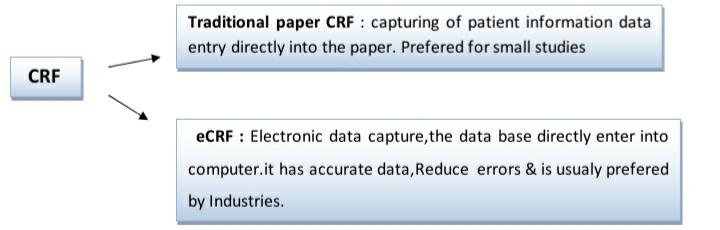
TYPES OF CRF :
THREE THINGS BEFORE WORKING ON CRF:
TYPES OF ITEMS:
Item questions control type item response
1. RADIO : Single selection [□Yes/ □ No]
Example : Gender : Male / Female
2. Check box : used for selecting one or more options
4. Pull down :
5. Date of time : Example : Date of birth : [02/AUG/2010] DD/MMM/YYYY
Time : Hr/min,
6. Integer :
7. Decimal values :
TYPES OF FORMS :
1. Dynamic form : A form which get triggered based on satisfaction of some criteria
Example : if Gender = Female then trigger pregnancy form
2. Repeating form ; Same data points repeated for more than once
Example: Adverse event form, Medication history and concomitant medication forms
3. Add entry : Adding entries to forms which has a repeating sections only some items are going to be repeated for it .particular assessments.
Example : Medical history form( MH,CM has particular section repeated in add entry)
4. Flat form : A Form called as flat form is the information which entered once about subject .the form wount be repeated.
Example: Demographic form
TYPES OF VISITS :
Dynamic visit : Add of subject visit at any time based on date of visit
Example : if a subject agree s for extention of participation ,extention visits generated automatically.
Repeating visits : A visit repeated ,undefined visit
Example : In oncology trials repeating of visits end point till death
Unscheduled visit : A visit scheduled not as per protocol
Example : subject with complain of adverse event unscheduled visit.
Scheduled visit : A visit which have been scheduled as per protocol
STANDARD CRF TEMPLATES :
1. Demographic form
2. Adverse event form
3. Vital Signs
4. Inclusion & exclusion
5. Concomitant form
6. Medication History
7. Pregnancy form
8. ECG form
9. Lab form
10. Disposition form
By,
Heena Ameena
Pharm D (Intern) Clinosol Research Pvt Ltd










Comments