CLINICAL TRIAL REGISTRATION
Clinosol is celebrating International clinical trials day-2020 to pay a tribute to the research contributions for public health. To know more about the event and exciting participation events like quiz, poster, essay writing and pledge for free, please visit www.internationalclinicaltrialsday.org or contact us at 9121151622/623/624 or write to us at clinicaltrialsday2020@gmail.com
CLINICAL TRIAL REGISTRATION
Clinical trial registration is the practice of documenting clinical trials before they are performed in a clinical trials registry so as to combat selective reporting and publication bias. ClinicalTrials.gov is the first online clinical trial registry and the largest clinical trial database run by the United States National Library of Medicine(NLM) at the National Institutes of Health which made registry available to the public via internet on 29 February,2000. International Committee of Medical Journal Editors (ICMJE) announced that only registered trials would be considered for publication.
Various clinical trial outcomes are not reported due to negative or equivocal results and some trial data and reports are often difficult to find or abandoned. However, duplicate publications, selective reporting and publication of research outcomes resulted in data misinterpretation and failure in providing evidence based medicine. Thus clinical trial registries are considered as essential to maintain transparency, accountability and accessibility of clinical trial data.
Clinical trial registries :
Registries are classified as primary and partner registries, using WHO International Clinical Trials Registry Platform(ICTRP). Currently, WHO registry network includes 16 primary registries.
• Primary registry is a clinical trial registry with atleast a national or regional remit that meets WHO registry criteria for quality, validity, accessibility, content, technical capacity, governance and administration. Primary registries are endorsed by the International Committee of Medical Journal Editors(ICMJE).
• Partner registry also satisfies WHO registry criteria but need not have a regional or national remit. Partner registries must be affiliated with either a primary registry in WHO registry network or an ICMJE approves registry.
Data providers provide data to WHO in the ICTRP search portal for maintaining database of the registries. Data is updated on particular time for any inclusions in the ICTRP search portal.
Eg : Clinical Trials Registry of India on every 4th week, Australian New Zealand Clinical Trials Registry on every Wednesday evening either every week.
Why is trial registration important ?
According to WHO, the registration of all interventional trials is a scientific, ethical and moral responsibility because :
• There is a need to ensure that decisions about health care are informed by all of the evidence.
• The Declaration of Helsinki states that 'every clinical trial must be registered in a publicly accessible database before recruitment of the first subject.'
• To ensure transparency, accountability and to increase public trust in the conduct of clinical research.
• Clinical trial registrations and results reporting would ensure unbiased public records on safety and efficacy of drugs.
• Registries checking data as part of the registration process may lead to improvements in the quality of clinical trials by identifying potential problems early in the research process.
• Improve awareness of similar or identical trials will make researchers and funding agencies to avoid unnecessary duplication.
Clinical Trials Registry of India (CTRI) :
The Clinical trials registry of India, is a primary registry hosted at ICMRs National Institute of Medical Statistics (NIMS). It is a free and online public record system for registration of clinical trials being conducted in India, launched on 20th july,2007 (www.ctri.nic.in) . CTRI is the first registry in Asia. It was initiated as a voluntary measure then Drugs Controller General of India(DCGI) made registrations mandatory from 15th june, 2009 (www.cdsco.nic.in). Editors of 11 biomedical journals declared that only registered trial reports would be published. Completed trials, trials in which patient recruitment has started (retrospective registration) are also being registered in CTRI. CTRI has announced that only prospective registrations (registration before the enrolment of first participant) will be allowed from 1st april, 2018. Trials conducted in other countries which do not have a primary registry can be registered in CTRI.
Objectives of CTRI :
• To increase awareness and accountability of all the participants of clinical trials.
• To promote training, assistance and advocacy for clinical trials by creating database and modules of study for various aspects of clinical trials and its registration.
• To provide a corrective system against positive results bias and selective reporting of research results to peer review publication.
• To create a complete, authentic and readily available data of all ongoing and completed clinical trials.
• To make information available to both public and healthcare professionals in an unbiased, scientific and timely manner.
• To establish a public record system by registering all clinical trials on health products including drugs, vaccines, devices etc.
What to register ?
All trials conducted in India, involving human subjects, randomized or not, of any interventions such as drugs, devices, surgical procedures, lifestyle changes, behavioural treatment etc. Trials being conducted in department of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homeopathy (AYUSH) are expected to be registered.
Clinical trials undertaken by postgraduate students for their thesis should be also registered.
Who registers clinical trial ?
It is the duty of the principal investigator (PI) to register a trial.
In multi-centric or multi-sponsored trial , lead investigator or lead sponsor should register the trial.
In multi-national trial, the Indian PI should register the trial in CTRI, mentioning the relevant registration numbers in registries of other countries.
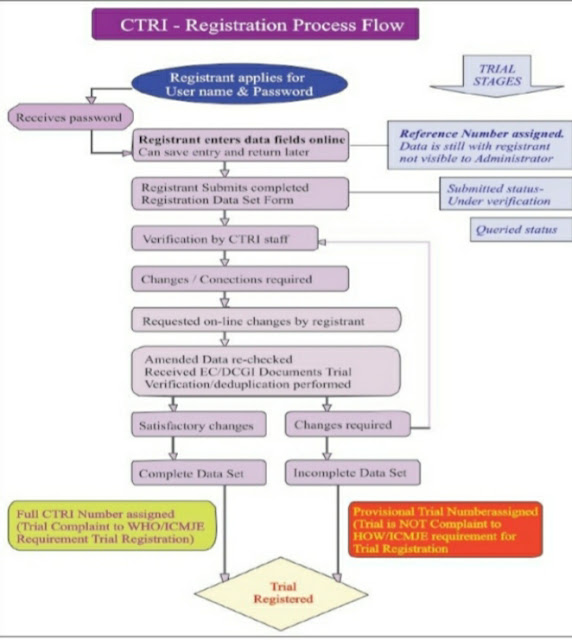
How to register a clinical trial in CTRI ?
login into CTRI website : www.ctri.nic.in
For registration of a trial, minimum information to be entered in the register is provided under 24 items in the WHO trial registration data set (Version 1.3.1-ICTRP). TRDS includes :
1. Primary registry and trial identifying number
2. Date of registration in primary registry
3. Secondary identifying numbers (Universal Trial Number (UTN), Identifiers assigned by sponsor, identifiers issued by funding bodies, regulatory authorities, ethics committees, institutional review boards, etc.)
4. Source of monetary or material support (funding agency, foundation, company, institition)
5. Primary sponsor
6. Secondary sponsor (additional individuals, organizations, other legal persons)
7. Contact for public queries (email address, telephone number, postal address)
8. Contact for scientific queries (details of principal investigator : name and title, email address,
telephone number, postal address etc)
9. Public title
10. Scientific title
11. Countries of recruitment
12. Health condition or problems studied
13. Intervention (name and description)
14. Key inclusion and exclusion criteria
15. Study type (type of study, study design, phase; for randomized trials : allocation method)
16. Date of first enrolment
17. Sample size
18. Recruitment status
19. Primary outcome
20. Key secondary outcome
21. Ethics review (status, date of approval, name and contact details of ethics committee)
22. Completion date
23. Summary results
24. IPD(individual participant data)sharing statement(what will be shared, with whom, when)
List of various countries clinical trial registries :
• India - Clinical Trials Registry -India (CTRI)
• USA - CinicalTrials.gov
• Australia and New Zealand – Australian and New Zealand Clinical Trial Registry (ANZCTR)
• China - Chinese Clinical Trial Registry (ChiCTR)
• European Union - EU Clinical Trials Register (EU-CTR)
• Germany - German Clinical Trials Register (DRKS)
• UK - ISRCTN.org
• Africa - Pan African Clinical Trial Registry (PACTR)
• Srilanka - Srilanka Clinical Trails Registry (SLCTR)
• Brazil - Brazilian Clinical Trials Registry (ReBec)
By-
A.V.Alekhya
(avalekhya1998@gmail.com)
K.Someswara Rao
(someshkarra95@gmail.com)
Pharm D V yr

Comments